
Ingenia
Therapeutics
Ingenia Therapeutics is a biotech start-up developing therapeutic antibodies aimed at restoring microvasculature in multiple ophthalmic and systemic diseases.
Expertise
Utilized
Protein engineering, protein expression and purification, SPR, functional cell based assays, PK/PD sample analysis
Ingenia Therapeutics tops current standard of care for degenerative retinal disease with modified bispecific antibody technology
PEG IGT-427
Simultaneous
dual function binding against Tie2 and VEGF
2X
increase in ocular half-life
Reverses
VEGF-induced endothelial barrier leakaga
Overview
Wet age-related macular degeneration (AMD) and diabetic macular edema (DME) are two leading causes of vision loss, together affecting more than 40 million people globally. Both disease states are driven in part by the upregulation of vascular endothelial growth factor (VEGF) as well as Angiopoietin-2 (Ang2). A recently approved bi-specific antibody targeting Ang2 and VEGF, Faricimab, reduces intersititial retinal fluid and retinal thickness, compared to standard care treatment (Aflibercept).
Despite the advantages of Faricimab, many DME patients do not respond completely to treatment.
Mosaic and Ingenia generated an improved bispecific pegylated antibody, IGT-427, that has dual function of VEGF inhibition and Tie2 activation. The improved activity of IGT-427 includes direct activation of Tie2 that exceeds Ang2 blockade in vitro, protection against Tie2 downregulation from proteases, and long-lasting half-life in the vitreous. These higher and longer-acting pharmacodynamics, may lead to increased patient response and decreased frequency of administration compared to current standard of care.
Method Highlights
Binding and Functional Assays:IGT-427 binding affinities to VEGF and Tie2 were assessed by SPR analysis (Biacore). Potency and soluble Tie2 assays were carried out in either HUVEC cells or CHO cells overexpressing human Tie2. Endothelial barrier integrity was assessed using a TEER (trans- endothelial electrical resistance) assay with CellZscope® (Nanoanalytics).
Protein Engineering:PEGylated IGT-427 was produced by engineering cysteines near the C-terminus of IGT-427 heavy chain. The two free cysteines in this variant of IGT-427 were reacted with 20 kDa linear or 40 kDa branched PEG-maleimide.
PK Analysis:To assess the ocular PK of IGT-427 and PEGylated variants, total drug levels were measured by ELISA in the vitreous of rabbits after intravitreal injection with 500 μg of aflibercept, faricimab, IGT- 427, 20 kDa linear PEGylated IGT-427, or 40 kDa branched PEGylated IGT-427.
Results
IGT-427 binds Tie2 and VEGF simultaneously
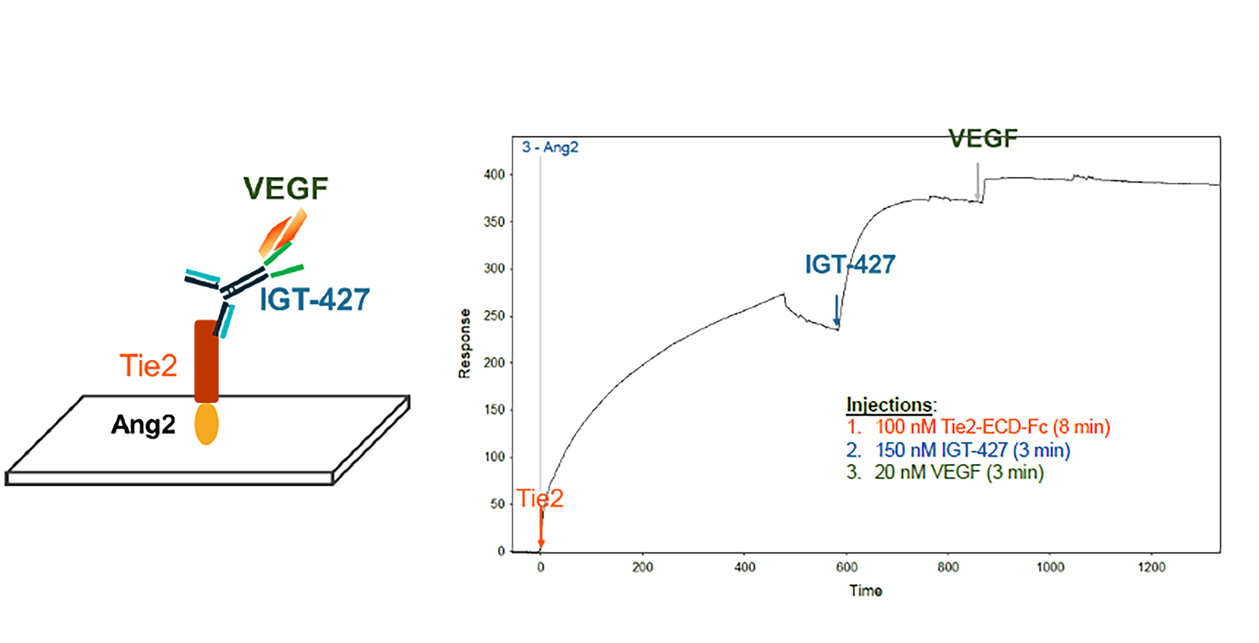
Surface Plasma Resonance (SPR), Biacore (Ang2 mobilized on a chip and sequential additions of
Tie2-ECD, IGT-427, and VEGF)
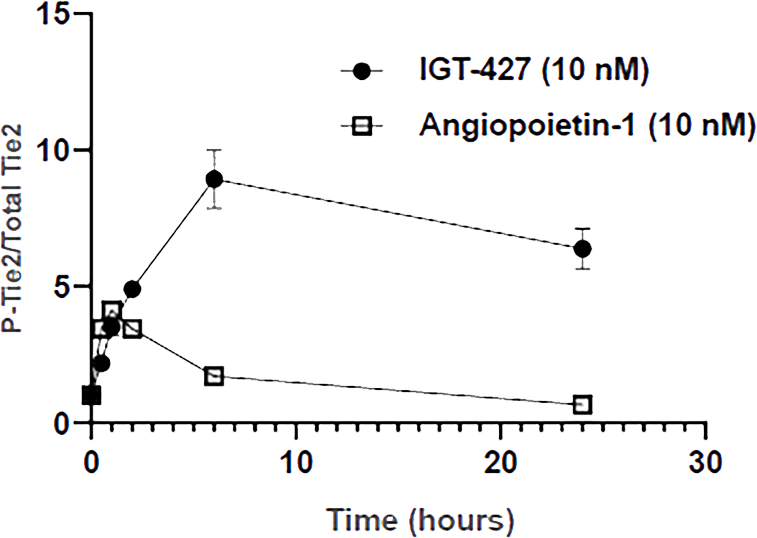
IGT-427 induces stronger and more durable activation of Tie2 than endogenous activator, Angiopoietin-1
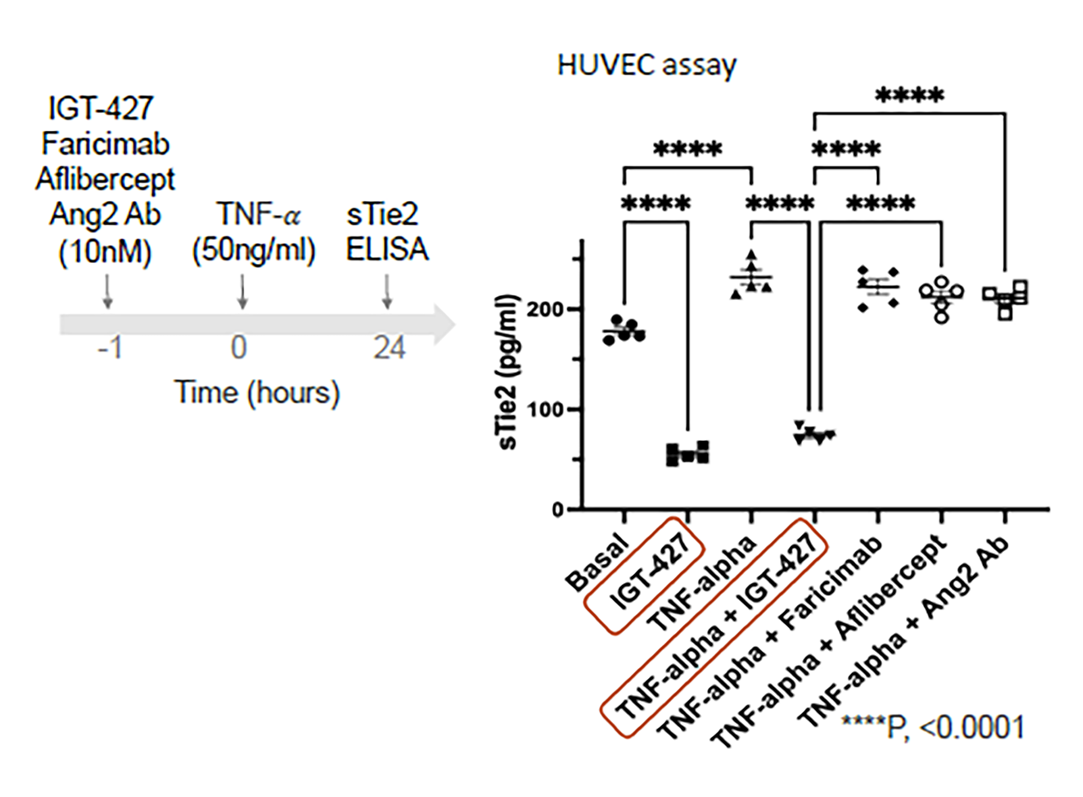
IGT-427 maintains beneficial signaling under inflammatory conditions (TNFα)
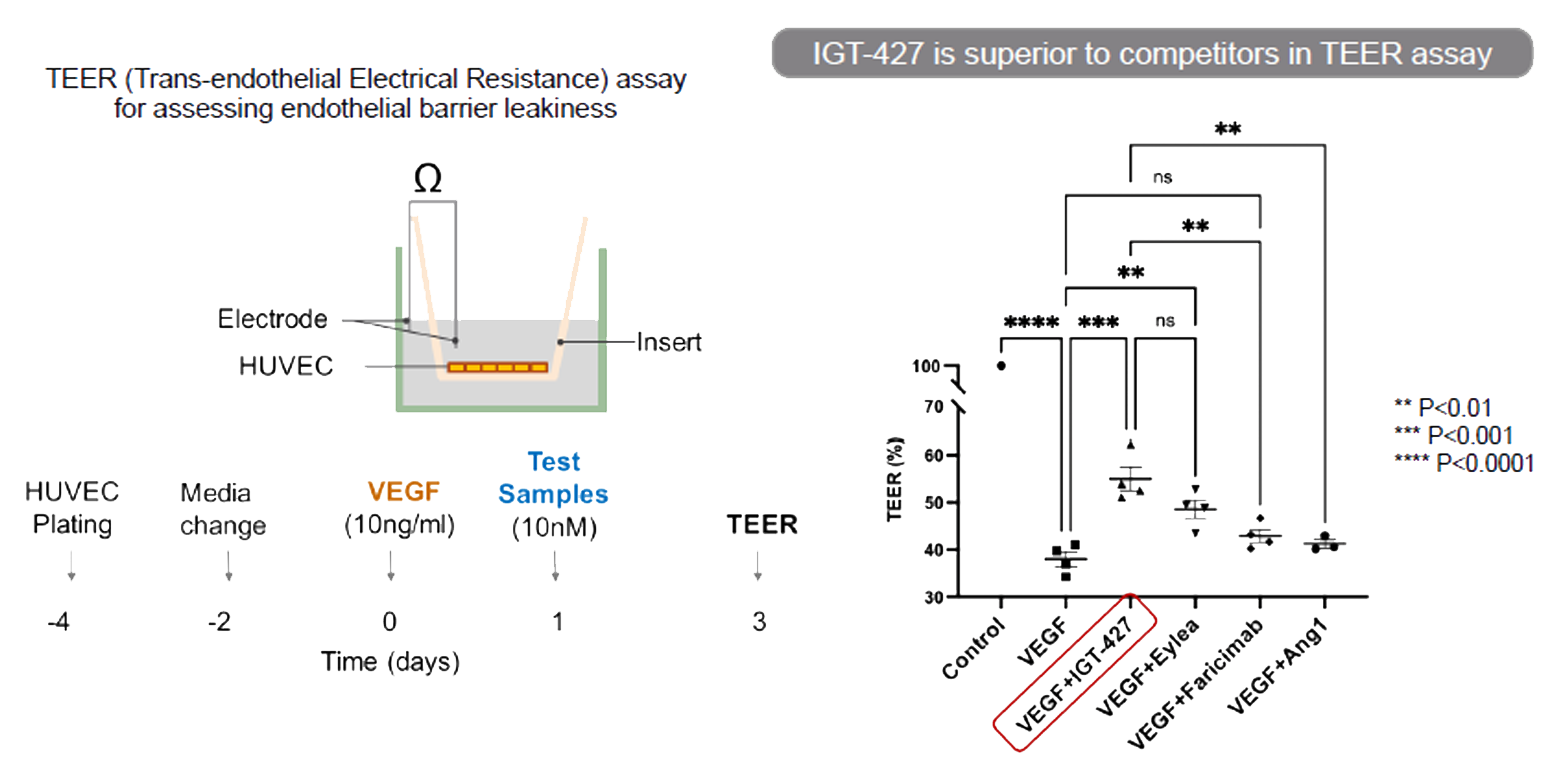
IGT-437 reverses endothelial barrier leakage introduced by VEGF
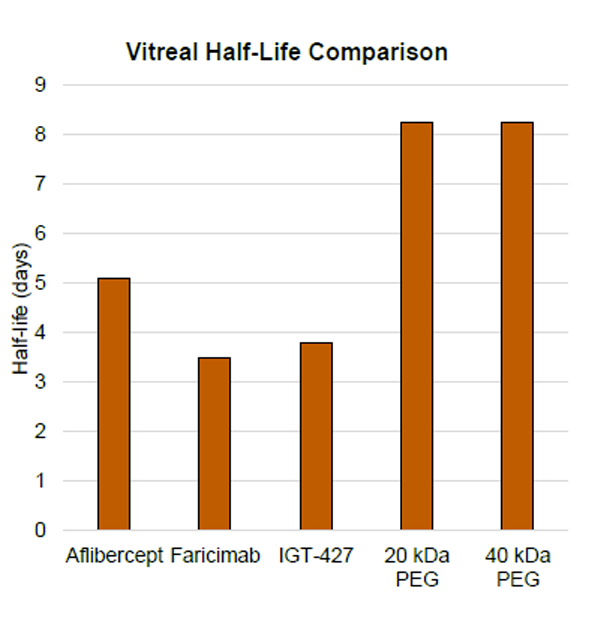
PEGylation of IGT-427 doubles the ocular half-life in rabbits
Conclusion: Compared to standard of care therapies, PEGylated IGT-427 provides comparable VEGF blockade, stronger Tie-2-mediated vasculature stabilization, and a longer vitreal half-life. These characteristics suggest that PEG IGT-427 has the potential to be a best-in-class treatment of AMD and DME, with greater efficacy and reduced frequency of administration.
Furfine et. al. (2022) "Variants of IGT-427 are long-acting, bispecific antibodies for the treatment of degenerative retinal diseases" Annual Meeting of the Association for Research in Vision and Ophthalmology